‘Africa isn’t a Testing Lab’: America’s Uncomfortable History with Drug Trials & Response to COVID-19
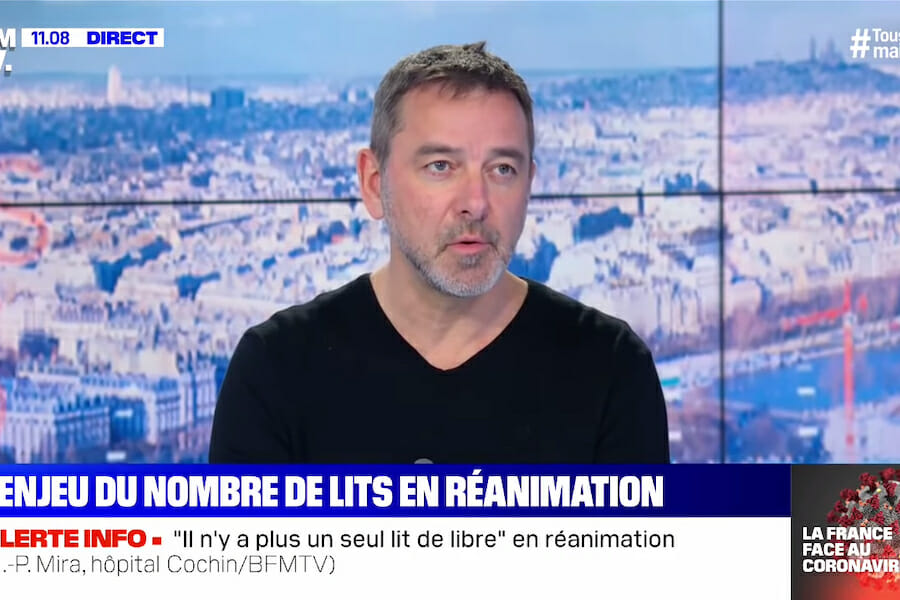
On April 3rd, Reuters reported an apology from a French doctor named Jean-Paul Mira for comments he made on a televised discussion suggesting that the testing of treatments for the global outbreak of COVID-19 should be conducted in Africa. His reasoning? He believed that conducting the studies where, in his words, “there are no masks, treatment or intensive care,” would produce more accurate results as to the ‘real’ rate of contagion of the virus and how various treatments respond to it.
To no one’s surprise, these callous comments were immediately, and rightly, called racist and were lambasted on social media. Along with former soccer star Didier Drogba, French researcher & Ph.D. candidate, Rim-Sarah Alouane, took aim at the comments on Twitter, saying, in part: “It’s 2020 in France & we still see people from Africa as subjects for experimentation. This is what normalized racism looks like. As far as I know, nobody jumped in to counter the arguments, both doctors agreed.”
Though Mira apologized, his comments bring into focus an uncomfortable reality. For decades, Western countries have used African populations, along with populations in other post-colonial nations, as guinea pigs for medical testing. Facing our current pandemic, it raises some pertinent questions about how the clinical trials for any coronavirus drugs will be handled.
It is totally inconceivable we keep on cautioning this.
Africa isn’t a testing lab.
I would like to vividly denounce those demeaning, false and most of all deeply racists words.Helps us save Africa with the current ongoing Covid 19 and flatten the curve. pic.twitter.com/41GIpXaIYv
— Didier Drogba (@didierdrogba) April 2, 2020
Currently, the largest study being conducted is a ‘megatrial’ being conducted by the WHO, called SOLIDARITY, which is recruiting patients from countries around the world. The study is testing 4 different drugs, and relies on reports from local hospitals, which, to a large extent, excludes countries without rigorous healthcare infrastructure in place. Though the WHO’s trial is the largest, it is not the only significant trial being conducted at this time. Two of the most promising trials are being conducted by familiar faces. Gilead, the drug company behind the horrific testing of the drug Tenofovir, is currently conducting a trial for their drug Remdesivir in Chicago, which is, admittedly, showing exciting results. And Pfizer? The company, in 2009, paid out a settlement of $75 million to the Nigerian state of Kano over deaths caused by their drug Trovafloxin. They recently announced they now intend to start human tests in August or September.
Once the crest of the outbreak is over, whenever that may be, the drugs that combat COVID-19 will continue to be researched, tested, and studied, even after they have left the public eye. The Food and Drug Administration Amendments Act (FDAAA) of 2007 requires sponsors of trials to report their results directly onto ClinicalTrials.gov within 1 year of completion- however, a study conducted found low compliance rates, along with little enforcement of the new rules. The only way for these companies to be held accountable for violations of human rights is with accurate data reporting, and that isn’t happening. Until we have enforced transparency of clinical trial data, we are only bound by a sense of trust that has been repeatedly broken by multinational pharmaceutical companies — and no human life should hang in the balance of companies quietly taking the advice of two French doctors’ racist brainstorming on live TV.
